Pharmaceutical Distribution Business
We are contributing to reduction in social costs by realizing a stable supply of pharmaceuticals with high distribution quality as social infrastructure and optimizing distribution inventory through the construction of a healthcare distribution platform.
Opportunities and risks
Opportunities
- Expansion of the specialty drug market
- Expansion of generic drug use
- Spread of digital technologies in the medical and nursing care fields
- Promotion of community-based healthcare collaboration and healthcare collaboration and comprehensive community care systems
- Reform of medical care in Asia
Risks
- Stagnant market growth due to controls over increases in national healthcare spending
- Changes in pharmaceutical distribution and sales activities (response/compliance with guidelines)
- Increased complexity of distribution inventory management
- Growing competition due to market entrants from other industries
- Natural disasters and pandemics
Suzuken Group strengths
Safe, secure, reliable pharmaceutical distribution system
- National scale, local focus
- Specialty drug traceability mechanism Cubixx
- High distribution quality compliant with global standards
Solid customer relationships
- Relationships of trust with customers through our “Smile Activities”
- Network connecting the Group with medical institutions, pharmacies, and healthcare professionals
- Reliable net sales and market share for ethical drugs
- Net sales of ethical drugs: 1.9779 trillion (FY2022)
- Domestic share: 22.29% (FY2022)
Business locations and shipping fleet of the Pharmaceutical Distribution Business(As of March 31, 2023)
- Business locations:209
- Wholesale distribution centers:15
- Shipping fleet:1,893vehicles
Main initiatives
Pharmaceutical Distribution Business
Safe, secure, reliable pharmaceutical distribution system
The Suzuken group purchases ethical drugs, diagnostic agents, medical equipment, medical materials, and medical food products from approximately 1,000 companies both in Japan and abroad; including pharmaceutical and medical equipment manufacturers, and supplies medical institutions and insurance pharmacies.


Reliable supply of the required quantity of drug at the required time
The Suzuken group has built a reliable network under strict quality control to deliver the required quantity of drug at the required time to every corner of the country. A nationwide traceability system centrally manages lot numbers and expiry dates and clarifies distribution channels for all pharmaceuticals. This ensures a quick response in the event of a drug recall.
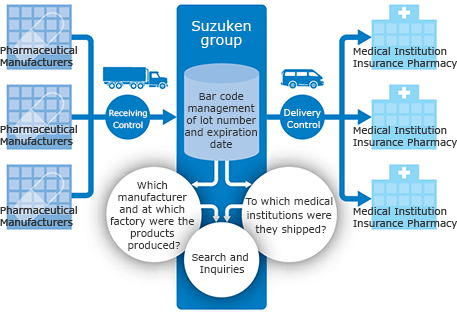
Stable supply system which also serves in emergencies
Beginning with distribution centers in 12 locations nationwide and 270 physical distribution bases, the Suzuken group has established a distribution system with an extensive inventory, ensuring that stock does not run out. In preparation for emergency situations such as earthquakes or other large-scale disasters or outbreaks of new strains of influenza, we work on maintaining a stable supply system on a daily basis.
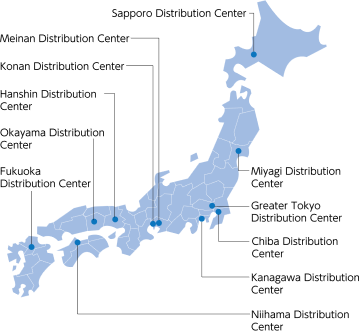


Evolution of healthcare distribution platform
Aiming to become Japan's No.1 medical logistics partner
The Suzuken group has strengthened its two functions of "Manufacturer distribution" and "Wholesaler distribution". These cover distribution from pharmaceutical manufacturers to pharmaceutical wholesalers and distribution from pharmaceutical wholesalers to medical institutions and insurance pharmacies respectively. We have also built a medical distribution platform with high-quality wide ranging functions unrivaled in the industry, including distribution of investigational and orphan drugs requiring strict temperature control.
In 2012, we became the first pharmaceutical wholesaler to offer comprehensive support services in the field of rare diseases. Using our healthcare distribution platform, we became able to flexibly respond to needs that could not be served by existing distribution systems and made the reliable supply of orphan drugs a reality.
Being the industry’s first provider of contract manufacturing distribution, through our track record as the industry’s top contractor in specialty drug distribution we have been able to build up a corps of human resources that have a wealth of experience and know-how, and these resources are the greatest strength of the Suzuken Group.
On the quality control front, the Suzuken Group currently operates 11 manufacturer distribution centers and six transportation terminals. Since 2008, nine distribution centers have obtained GMP※1 -compliant ISO9001 certifications. They later upgraded their certifications to the 2015 version to conform to the PIC/S※2 GDP※3 global standards, abide by a new set of operating standards known as the Good Distribution Practice Guidelines, and go to other lengths, as well, to achieve even stronger quality control. Appreciation for these strengths is leading to an increase in contract distribution for pharmaceutical manufacturers and specialty drugs.
-
※1GMP (Good Manufacturing Practice): Manufacturing and quality management guidelines for pharmaceuticals
-
※2PIC/S: A combination of abbreviations for the Pharmaceutical Inspection Convention (PIC) and the Pharmaceutical Inspection Co-operation Scheme (PICS), which are two international cooperation organizations that aim to improve cooperation among governments and inspection authorities in the areas of GMP and GDP
-
※3GDP (Good Distribution Practice): Quality management guidelines for the transportation and storage of pharmaceuticals
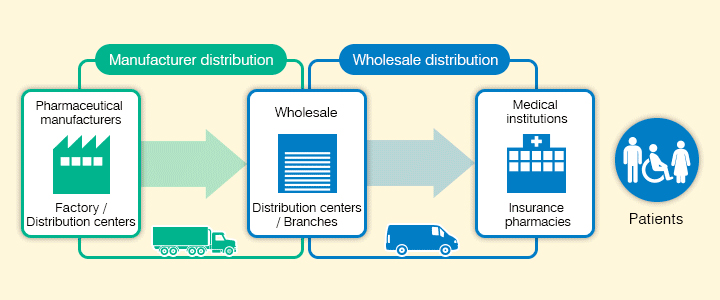

New distribution model for revised product categories
Specialty drug distribution
Since 2017, we have been deploying Cubixx, a specialty drug traceability system. This system uses RFID, which reads and writes data through IC tags, and dedicated storage cabinets equipped with IoT technology to enable 24/7 real-time remote monitoring of pharmaceutical management conditions, from the drugs’ transit to hospital storage, as well as the reverse distribution of returned items and their re-delivery. We have expanded our lineup thus far to include a hospital version, pharmacy version, clinical-trial version, home-use version, large-size version, and room-temperature version.
Monitoring pharmaceutical transit and storage conditions makes it possible to make resale decisions and ascertain inventory conditions, and it contributes to reducing pharmaceutical waste and preventing maldistribution of Construction of healthcare distribution platform utilizing digital means Social issues Reducing social costs by building a healthcare distribution platform utilizing digital means 33 Preparation for New Growth Businesses Strategy Implementation New mechanism enabled by inventory visualization Delivery schedule app, delivery schedule notification service Ordering support Automatic route creation Forecasts future demand based on delivery records, and alerts to orders and returns Allows online checking of the delivery date for ordered products, as well as the availability of substitutes Creates optimal delivery routes for each day, including returns and collections inventory. It also enables automated ordering and allows automation of things like proposals to refresh non-moving inventory or unused inventory, which helps to reduce the workload on medical institutions.
The Cubixx system has been adopted by 375 facilities throughout Japan as of the end of March 2023. Over the coming three years, we aim to expand its adoption to more than 600 facilities, primarily cancer hospitals.
Responding to needs for community-based healthcare collaboration and comprehensive community care systems
The Suzuken Group is working to connect people and resolve issues in local healthcare and nursing care through partnerships with pharmacies and nursing care businesses, and collaborations with IT-driven healthcare companies. Already, we have entered into partnership agreements with numerous local governments at the branch, sales department, and company level in an effort to move forward with contributions to local communities.

Healthcare Distribution Reform
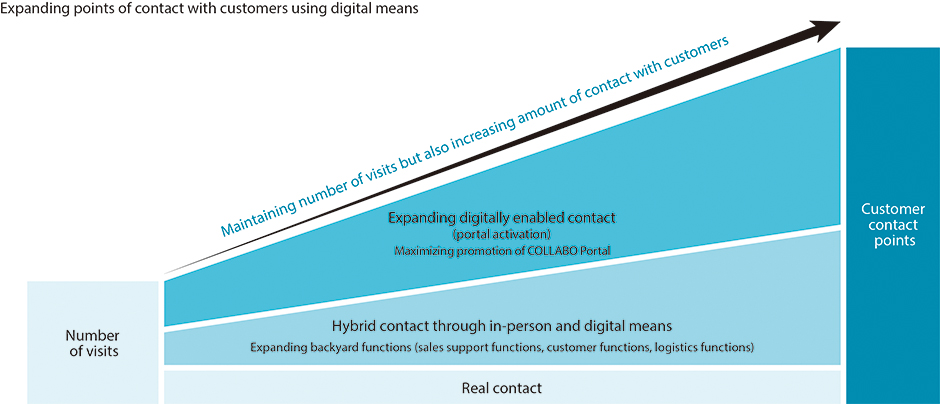
Combining real and digital means to increase customer contact points
The conventional pharmaceutical wholesale system relied on sales representatives known as MS, constantly requiring their judgment and confirmation for everything from orders to deliveries and returns. Considering that MS will be more important with the transition to a health creation enterprise, we will conduct healthcare distribution reform and build an effective and efficient sales mechanism to transition from pharmaceutical wholesaling to healthcare distribution.
Specifically, we will strengthen three backyard functions: the “Sales support functions,” by building a support mechanism that takes over the indirect operations of MS and also by utilizing the services of COLLABO Portal; “Customer functions,” by utilizing an app that visualizes inventory data and delivery schedules, and by building a customer center; and “Logistics functions,” by partnering with our Group company S.D. Logi CO., LTD. This will help create an environment where MS can concentrate on their original roles of connecting the Group and its customers, widening the scope of health creation, and creating revenue.
When it comes to sales activities, we will continue to value the conventional way of building relationships of trust with customers through visit-based, in-person sales, while also expanding digital points of contact, such as by providing information and proposing solutions through COLLABO Portal. While optimizing the number of visits, we will also emphasize efficiency and maintain the amount of contact points with customers.
Main solutions resulting from collaborations
- The Cubixx specialty drug traceability system
- PS+Management inventory management system for pharmacies
- PS+Voice automatic voice recognition and electronic medication history system
- Medifiims PE asset management system for pharmacies
- ES Navigation, a remote detail service
- Digital services: Medical Care Station (MCS), a communication tool dedicated to medical and nursing care professionals Dr. JOY medical institution support platform, Welby MyKarte PHR (Personal Health Record) platform, Ubie for Hospitals AI-based medical interview service, SmartMat, an IoT order and inventory management solution
- ARUU, a prescription drug delivery service
Constructing new sales mechanisms
By introducing digital and backyard functions into our sales activities, we will construct two sales mechanisms: “hold-fast sales” via the healthcare distribution platform that acts as social infrastructure, and “new sales” that provide new value. For “hold-fast sales,” we will leverage the relationships of trust cultivated thus far with customers, along with the Suzuken Group’s ability to mobilize, to help with problem solving on the front lines for customers and communities.
At the same, we will promote COLLABO Portal to fulfill the coordinator role of connecting the Group with customers and communities. In “new sales,” we will propose solutions to key personnel in medical and nursing care and the community utilizing digital health services posted on COLLABO Portal.
In the future, we aim to provide new value and contribute to not only medical and nursing care professionals, but local society as well.
Asian business
Rollout of pharmaceutical distribution business in China and South Korea
Constructing medical platform in China
In Asia, national governments are moving ahead with healthcare system reforms in response to their growing populations and rising economic performance. The implementation of advanced, efficient pharmaceutical distribution is a high priority and there are great expectations for how the Suzuken Group, and the experience and knowledge it has developed in Japan, can contribute.
China, a country of particular interest, with developments such as rising incomes and the institution of a healthcare insurance system, is now the world’s second largest pharmaceutical market.
Suzuken has been developing pharmaceutical distribution operations in Shanghai and Qingdao by establishing in 2008, a joint venture (49.9% ownership), Shanghai Suzuken Huzhong Pharmaceutical Co., Ltd. (now SPH Suzuken Huzhong (Shanghai) Pharmaceutical Co., Ltd.), with Shanghai Pharmaceutical Co., Ltd., a major Chinese pharmaceutical wholesaler.
Furthermore, in September 2016, Suzuken acquired a 35% stake in EPS EKISHIN Co., Ltd.,※6 a member of the EPS Group. Together, the two are helping Suzuken (Shenzhen) Pharmaceutical Co., Ltd. (20% stake held by Suzuken) to introduce the products of Japanese pharmaceutical companies to the Chinese market and are performing technical sales promotion activities throughout China on behalf of Suzuken (Shenzhen) Pharmaceutical.
Working with its partners, Suzuken draws on R&D, clinical trial, manufacturing, sales, sales promotion, distribution, and collection functions to create a healthcare distribution platform capable of providing customers with the one-stop services they need. Looking to the future, Suzuken will continue to create new added value for the field of healthcare in China.

-
※6EPS EKISHIN Co., Ltd. is a specialized trading company connecting Japan and China in the field of healthcare. Its wide-ranging business pursuits span areas as varied as CRO services, pharmaceutical manufacturing, and sales agency for digital diagnostic imaging equipment.
Constructing national distribution network for healthcare products in South Korea
In South Korea, as in other countries, healthcare expenditures are on the rise and there are expectations that the healthcare industry will continue to advance and that the government will lead policy efforts to curb healthcare expenditures.
Suzuken entered into a capital and business alliance with BOKSAN NICE Co., Ltd.※7 taking a 45% stake in that company in June 2016. Drawing on knowledge it has developed in the field of health creation in Japan, the Suzuken Group is supporting BOKSAN NICE in its efforts to expand its pharmaceutical distribution business to cover all of South Korea and advance joint R&D in medical-care related businesses. The Suzuken Group is also helping BOKSAN NICE to enhance its corporate value through joint projects for strengthening its distribution functions and management platform.
By continuing to partner with local companies, we are also contributing to the advancement of pharmaceutical distribution and industries related to medical care in South Korea.

-
※7BOKSAN NICE Co., Ltd. with operations mainly in Busan Metropolitan City and the Seoul Capital Area, is a pharmaceutical distribution leader in South Korea.
Expanding existing services and discovering new services with Cencora
With the aim of expanding business in Asia, primarily in China and South Korea, we are deepening our partnership with major US wholesaler Cencora (former AmerisourceBergen) and considering the possibility of rolling out the Cubixx system. Also, through a partnership between our Corporate Venture Capital (CVC), which we established in 2022 to facilitate the Suzuken Group’s investment in health-tech companies, and Cencora’s CVC, AB Health Ventures ($150 million), we will discover new technologies and solutions in the global healthcare field and consider their introduction in Japan and Asia.
Related group companies and affiliates
Subsidiaries
Pharmaceutical Distribution Business
- Suzuken Co., Ltd.
- Sanki Corporation
- ASTIS Co., Ltd.
- Shoyaku Co., Ltd.
- Suzuken Okinawa Yakuhin Co., Ltd.
- Nakano Yakuhin Co., Ltd.
- Suzuken Iwate Co., Ltd.
- S.D.Logi CO., LTD.
- Cloumd Corporation
- PSC Co., Ltd.
- Sanki MediHeart Limited
Associated Companies
Asian Business
- SPH Suzuken Huzhong (Shanghai) Pharmaceutical Co., Ltd
- BOKSAN NICE Co., Ltd.
Business Overview (Four Businesses Supporting Health Creation)
Go to the top of “Business Overview (Four Businesses Supporting Health Creation)”
