Healthcare Product Development Business
We conduct research and development, manufacturing, and sales of ethical drugs, diagnostic reagents, and medical devices and materials that contribute to improvement of the quality of life of patients and unmet medical needs.
Opportunities and risks
Opportunities
- Change in disease pattern due to aging population and other factors
- Response to unmet medical needs
- Growth of outsourcing needs among pharmaceutical companies
- Increase in venture companies of various types and in new outsourcing service providers
- Rollout of electronic prescriptions
- Advance of digitalization in the medical and healthcare fields
- Diversifying needs for in-home medical treatment and nursing care
Risks
- Drug pricing system reform brought on by rising healthcare expenditures
- Advancement of globalization and intensification of competition
- Compliance with sales information provision guidelines
- Environmental pollution from R&D activities
- Growing competition due to market entrants from other industries
Suzuken Group strengths
Presence in the field of diabetes therapies and kidney dialysis
- Development and manufacturing of pharmaceuticals, diagnostic reagents, and diagnostic devices
Efficient, high-quality production technology
- Supply chain management system that ensures a reliable supply of high-quality products
- Advanced production system that is compliant with international GMP standards
- Track record in contract manufacturing for major pharmaceutical companies
Healthcare product development business locations
- Pharmaceutical manufacturing plant (Kumamoto)
- Research lab (Mie Research Park)
ISO13485 certification for medical equipment and quality management systems
Main initiatives
Pharmaceutical Manufacturing Business
Boosting our presence in diabetes therapies and kidney dialysis
We develop, manufacture and sell pharmaceuticals that contribute to the improvement of lifestyle-related diseases with a focus on diabetes, high added value generic drugs, blood glucose self-monitoring devices and diagnostic agents with a top share of the domestic market. Some of our factories conduct contract manufacturing for major pharmaceutical manufacturers. Sanwa Kagaku Kenkyusho's own formulation technology has earned high evaluation at home and abroad.

Manufacturing of ethical drugs
At Sanwa Kagaku Kenkyusho Co., Ltd. we give serious consideration to patient QOL and economic aspects, and develop easy-to-use, safer and more effective medicines.



Manufacturing of diagnostic agents
At Sanwa Kagaku Kenkyusho Co., Ltd. we manufacture essential high-quality diagnostic drugs and diagnostic equipment for ultrasonography, electrocardiography and for testing blood and urine specimens in hospitals and clinics.

Contract manufacturing of pharmaceuticals
At Sanwa Kagaku Kenkyusho's Kumamoto Factory, we carry out contract manufacturing for major pharmaceutical manufacturers in addition to manufacturing our own pharmaceuticals.
Investment to further expand development pipelines
As a pharmaceutical manufacturer, our ongoing growth relies on the development of new pharmaceuticals. We view diabetes, and kidney and dialysis-related areas, as areas with substantial unmet medical needs and strong potential as new growth markets. These areas are at the center of our efforts to develop new pharmaceuticals and enhance our pharmaceutical development pipeline.
In August 2021, we launched the UPASITA IV Injection Syringe for Dialysis, a therapeutic agent for secondary hyperparathyroidism, and we are co-promoting it with Kissei Pharmaceutical Co., Ltd., in an effort to penetrate the market. In February 2022, we concluded a license agreement with Crinetics Pharmaceuticals, Inc.※1 relating to the exclusive development and commercialization of Paltusotine※2 in Japan, and we will move forward on development.
-
※1Crinetics Pharmaceuticals, Inc.: A clinical-stage pharmaceutical company that focuses on discovering, developing, and commercializing new therapies for people with rare endocrine diseases or endocrine-related tumors
-
※2Paltusotine (tentative name): A nonpeptide, orally bioavailable agonist that has high somatostatin receptor 2 (SST2) selectivity and curbs growth hormone secretion
New business creation through integration of Group functions and collaborations
We are reviewing and restructuring Sanwa Kagaku Kenkyusho’s supply chain as a whole. Taking into account factors such as the direction of new drug development and the increasingly globalized business environment, in September 2022, we transferred the Fukushima Factory, a pharmaceutical manufacturing business location, to Bushu Pharmaceuticals Ltd., which has acquired GMP certification in several countries around the world. We will be jointly employing sophisticated technology and production operations.
Drawing on the strengths we have cultivated as a pharmaceutical manufacturing—high quality, reliable supply, and low cost—the Suzuken Group aims to create new business models and enhance productivity by combining these strengths with its other capabilities and collaborating with partner companies.
Medical Equipment Manufacturing Business
Pursuing the development of products that are "patient-friendly", developing globally
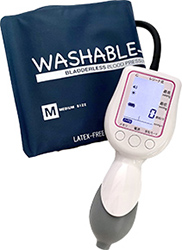
Our core medical equipment whose brand name is "Kenz" developed by Suzuken group is testing equipment such as Holter electrocardiographs, blood pressure monitors and stethoscopes. We are boldly taking on challenge to develop the equipment to make testing easier and more accurate as well as no burden and anxiety to patients and have realized the miniaturization of Holter electrocardiographs.
We have constructed systems including the "ISO13485" quality assurance system for medical equipment to meet global quality requirements. Kenz products are used in medical practice in over 30 countries worldwide, mainly in the Asian region, and have received high evaluation from medical institutions around the world.

Expansion of medical equipment and supplies manufacturing field, and strengthening of our competitiveness in it
Kenzmedico Co., Ltd. leverages the Group’s sales marketing capabilities to develop and manufacture not only the Kenz-brand medical devices and supplies sold through Suzuken but also medical devices such as stethoscopes and sphygmomanometers for professional use. In February 2022, we obtained medical equipment certification and insurance coverage for the “Simple Holter,” a test kit that integrates a single-use waterproof Holter electrocardiograph and an electrocardiogram data analysis service. This service is easy to use even at medical institutions that do not have testing experience.


Related group companies and affiliates
Subsidiaries
Pharmaceutical Manufacturing Business
Medical Equipment Manufacturing Business
Business Overview (Four Businesses Supporting Health Creation)
Go to the top of “Business Overview (Four Businesses Supporting Health Creation)”
